Explainer: 15 seconds that save lives: Inside Iran’s world-first cancer surgery breakthrough
By Ivan Kesic
In the quiet urgency of a surgical suite, where precision determines outcomes, Iranian innovators have developed a device that redefines speed and accuracy in the fight against breast cancer.
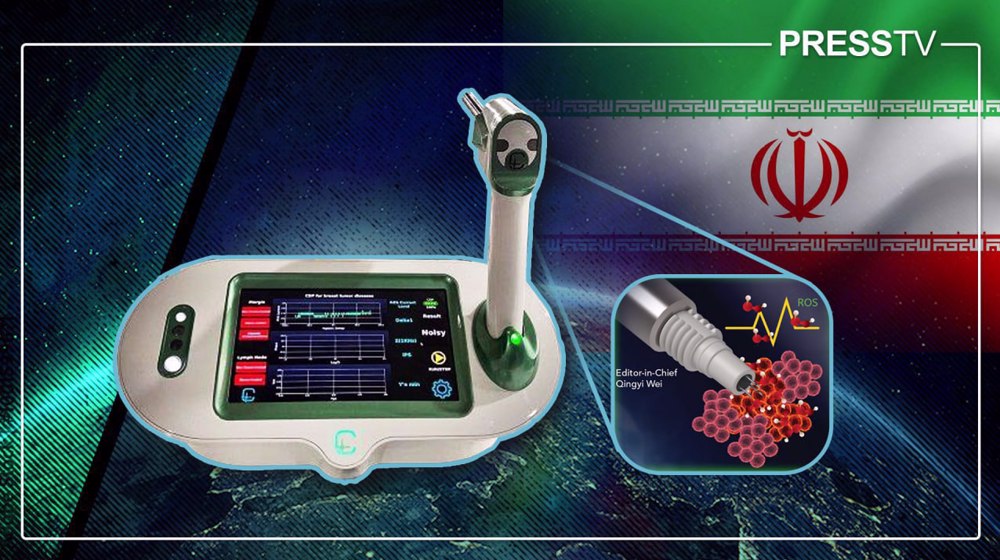
The Cancer Diagnostic Probe (CDP) is a handheld instrument born from years of interdisciplinary research, enabling surgeons to see what was once invisible or impossible.
In just 15 seconds, it can detect microscopic cancer cells that may remain after tumor removal, providing real-time insight during surgery.
The CDP’s journey, from a fundamental discovery in cancer cell metabolism to a clinically validated tool now used in hospitals across Iran, is a compelling example of scientific translation into practice and how innovation can help improve and save lives.
Powered by advanced biochemical principles, the probe achieves an impressive 93 percent accuracy, a performance substantiated by robust clinical data from multiple human studies.
Beyond its technical success, this innovation signals meaningful progress for the future of surgical oncology and underscores the growing importance of technological self-reliance in healthcare.
What is the core challenge in breast cancer surgery?
The primary objective of breast-conserving surgery (lumpectomy) is to completely remove the tumor while preserving as much healthy tissue as possible.
A critical determinant of success is achieving clear margins, confirming that the outer edges of the excised tissue are free of cancer cells.
When cancerous or pre-cancerous cells remain at the surgical cavity’s edge, the risk of local recurrence rises substantially, often leading to a second, more extensive operation and the need for more aggressive follow-up treatments.
Globally, statistics show that approximately one in five women experiences a recurrence after initial breast cancer surgery.
Traditionally, surgeons have relied on intraoperative frozen section analysis to assess margin status. In this approach, samples from the surgical margins are sent to a pathology lab, where the tissue is rapidly frozen, sliced, stained, and examined under a microscope.
While clinically valuable, this method has significant limitations. The process can take up to 45 minutes, prolonging the patient’s time under anesthesia.
Moreover, pathologists evaluate only selected, thin sections of the margin, increasing the risk that dispersed cancer cells may go undetected. As a result, frozen section analysis typically achieves a diagnostic accuracy of only 70-88 percent.
Studies have shown that even with frozen section analysis, up to 40 percent of involved margins can be missed. The challenge is even greater in patients who have received neoadjuvant chemotherapy, as treatment-induced tissue distortion and shrinkage further complicate microscopic evaluation.
This persistent gap between surgical need and available technology ultimately set the stage for the development of the Cancer Diagnostic Probe (CDP), made in the Islamic Republic of Iran.

How does the CDP work?
The innovation of the CDP lies not in imaging tissue structure, but in detecting live metabolic activity. It is grounded in a fundamental property of cancer cells known as the Warburg effect, also referred to as hypoxia-assisted glycolysis.
Even in the presence of oxygen, cancer cells preferentially shift their energy production to glycolysis, a less efficient pathway that generates elevated levels of lactate and reactive oxygen species (ROS), including hydrogen peroxide (H₂O₂).
This altered metabolic state is a defining hallmark of malignancy and is present even in pre-cancerous cells. The CDP leverages this biological signature through an electrochemical biosensor capable of detecting metabolic activity in real time.
The system comprises three core components. The first is a disposable needle sensor coated with nanostructured materials, such as multi-walled carbon nanotubes, which functions as the electrochemical detector.
The second is a wireless, handheld probe that allows the surgeon to position the sensor precisely against tissue. The third is a central control unit that processes the electrochemical signals and displays the results.
During surgery, once the primary tumor has been excised, the surgeon applies the probe to multiple points along the cavity wall. The needle sensor penetrates only a few millimeters into the tissue, where it measures the local concentration of ROS – specifically H₂O₂ - released by metabolically active cells.
Within approximately 15 seconds, the system analyzes the signal and categorizes the tissue on a monitor as Negative, Suspicious, or Positive, providing an immediate, metabolism-based assessment of surgical margins.
To ensure patient safety and eliminate the risk of cross-contamination, the probe head is single-use and replaced for each patient.
🇮🇷 First of its kind in the world:
— Press TV 🔻 (@PressTV) December 22, 2025
Iranian knowledge-based company makes novel surgical assistant device.#IranFirst pic.twitter.com/pDV0xGSamt
What does the clinical evidence show?
The promise of the CDP is supported by rigorous clinical research published in peer-reviewed international journals.
Across multiple studies involving hundreds of patients, the CDP has demonstrated strong clinical performance, both as a complementary tool alongside frozen section analysis and as a potential standalone guide for intraoperative decision-making.
A pivotal study in patients with non-neoadjuvant breast cancer reported compelling results when CDP measurements were benchmarked against post-operative permanent pathology, the clinical gold standard. The study demonstrated an overall sensitivity of 97 percent, specificity of 89.3 percent, and an accuracy of 92 percent.
Importantly, the research program was designed to address specific, real-world clinical questions. In one observational study, the CDP independently identified margin involvement with high accuracy.
In a subsequent interventional study, the probe detected cancers that had been missed by frozen section analysis, achieving a sensitivity of 93.8 percent for these otherwise overlooked lesions.
Another interventional study highlighted the CDP’s ability to support pathologists directly. When CDP findings prompted re-examination of frozen section samples, diagnostic errors were reduced, underscoring the probe’s value as a decision-support tool.
Even more striking evidence emerged from a later study examining cases in which the CDP detected cancer at the cavity wall while the corresponding tumor-side margins were reported as clear by permanent pathology, the most comprehensive form of histological assessment.
Among cavity-side lesions flagged by the CDP, numerous cases of invasive carcinoma or ductal carcinoma in situ (DCIS) were not documented in the tumor-side permanent pathology reports.
These findings suggest that the CDP can identify satellite cancer cells that have migrated beyond the primary tumor mass, cells that would otherwise remain undetected and could lead to recurrence, since no re-operation would be indicated based on a “clear” permanent pathology result.
Finally, a separate study focusing on patients who had undergone neoadjuvant chemotherapy confirmed that the CDP maintains a high sensitivity of 91 percent even in these diagnostically challenging cases, where frozen section analysis is known to be less reliable.
Iranian researcher wins gold at Silicon Valley invention festival for cancer medicine#IranFirst🇮🇷https://t.co/IDNC6Z04Lw
— Press TV 🔻 (@PressTV) December 16, 2025
From concept to clinic: the path of Iranian innovation
The development of the CDP is the result of a sustained, interdisciplinary effort spanning nearly a decade. The project was led by Professor Mohammad Abdolahad and his team at the Nano Bio Electronic Devices Laboratory within the School of Electrical and Computer Engineering at the University of Tehran.
Professor Abdolahad spearheaded the translation of a foundational bioelectronic concept into a practical surgical tool. This work was carried out in close collaboration with oncologists, surgeons, and pathologists from major cancer institutions, including the Cancer Research Center of Shahid Beheshti University and the Breast Cancer Research Center at the Motamed Cancer Institute.
The core scientific principles and early sensor technology were protected at an early stage of development. To date, the CDP is supported by multiple US patents, underscoring its originality and international relevance.
The path from initial proof of concept to a clinically deployed, mass-produced medical device took approximately eight years. This process included extensive preclinical animal studies followed by multi-phase human clinical trials to validate safety, performance, and clinical utility.
Commercialization was achieved through the knowledge-based company Nano Hesgarsazan Salamat Arya, which now manufactures the device domestically. The CDP has received full medical approval from the Iranian Ministry of Health and is actively used in surgical settings.
According to recent reports, the device has been delivered to leading hospitals across Iran, including Shohada Tajrish Hospital and Imam Khomeini Hospital in Tehran.
As a result, advanced, real-time margin assessment during breast-conserving surgery has become a practical, homegrown reality within the country’s healthcare system.
✍️ Iran First - Iran’s unstoppable ascent to global medical-tech dominance amid sanctions
— Press TV 🔻 (@PressTV) December 12, 2025
By @kesic_ivan
Read More: https://t.co/1tjthKJp0Y pic.twitter.com/OrxTtHUrs8
What does the CDP mean for patients and the future of surgery?
The immediate impact of the device is most evident in the operating room. By delivering real-time, high-accuracy feedback, the device enables surgeons to make better-informed intraoperative decisions.
Its potential to reduce the incidence of positive surgical margins by approximately 30 percent could have far-reaching clinical benefits. These include fewer repeat surgeries, lower rates of local recurrence, reduced patient anxiety, and improved cosmetic outcomes made possible by more precise, tissue-sparing excisions.
For Iran’s healthcare system, the CDP represents a major step toward technological self-reliance.
It is recognized as the world’s first fully Iranian-developed cancer surgery device of its kind. Developing and manufacturing the technology domestically ensures reliable access to advanced care while maintaining control over production costs, maintenance, and future upgrades, free from the vulnerabilities of international supply chains or external restrictions.
The future trajectory of CDP technology is broad and ambitious. Researchers are actively pursuing regulatory approvals to extend its application to other cancers, including thyroid and gastrointestinal tumors.
More broadly, the underlying platform of electrochemical metabolic sensing is opening pathways to what some researchers describe as electrotechnical onco-surgery, a new paradigm that integrates real-time metabolic diagnostics into surgical practice.
The innovation has also attracted international interest, with early discussions underway regarding deployment in hospitals outside Iran.
A new paradigm in cancer care
The CDP is more than a medical device. It represents validation of a scientific approach that views cancer through the lens of cellular metabolism.
By addressing one of the most persistent challenges in surgical oncology – the real-time identification of clear margins – Iranian scientists and engineers have made a meaningful contribution to global cancer care.
The CDP’s journey from university laboratories to operating rooms, supported by robust clinical evidence and protected by international patents, stands as a compelling example of how focused, interdisciplinary innovation can improve patient outcomes and reshape surgical standards.
As the technology continues to evolve and expand into new clinical applications, it reinforces the power of locally developed solutions to meet universal healthcare challenges.
VIDEO | Axis of Resistance stands as multinational front for justice
Swiss academics call for end to research treaty with Israel over Gaza genocide
VIDEO | Israeli regime harasses, tortures Gazans returning through Rafah crossing
Israel faces existential threat of internal collapse before centenary, general says
Police fire tear gas as protests erupt against ICE and Israel at Milan Winter Olympics
UK PM’s chief of staff resigns over appointment of Epstein associate as US envoy
Iran leads Islamic world in electric vehicle motor technology
VIDEO | Pakistan lawmakers reflect on legacy of Iran’s Islamic Revolution












 This makes it easy to access the Press TV website
This makes it easy to access the Press TV website