Iran starts third clinical trial phase of homegrown recombinant vaccine Razi Cov Pars for COVID-19
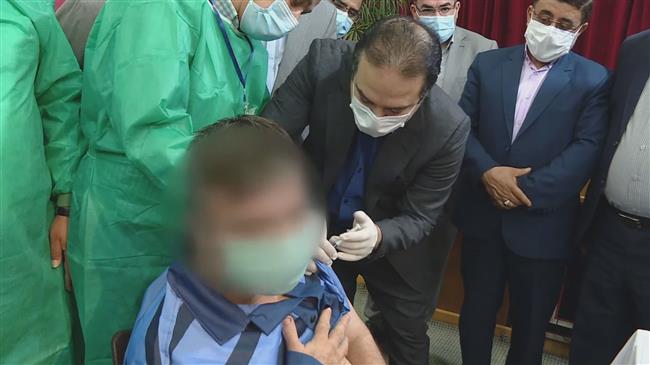
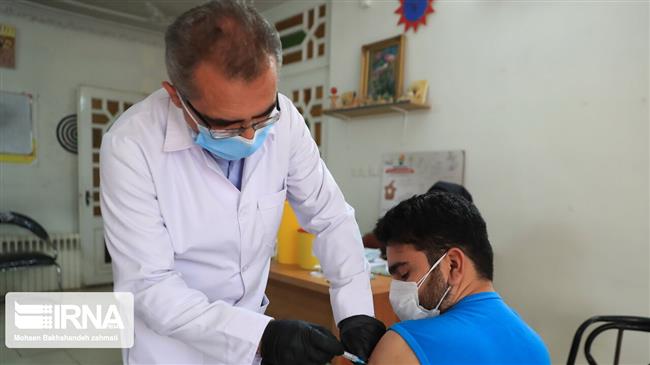
Iran has started the third phase of the human trial of its second homegrown coronavirus vaccine, Razi Cov Pars, less than a week after Health Minister Bahram Einollahi said accelerating the pace of vaccination against COVID-19 is the top priority of the new administration.
Mohammad Hossein Fallah Mehrabadi, vice president for research and technology affairs at the parent Razi Vaccine and Serum Research Institute and spokesman for the project, said on Sunday that the phase will be carried out in Tehran and Alborz provinces with the participation of some 40,000 volunteers.
People aged 18 or older, who have not contracted the coronavirus, are eligible to take part in the trial, he said.
According to Fallah Mehrabadi, the Razi Vaccine and Serum Research Institute produced 400,000 doses of the vaccine last month. The minister hoped that his institute would manufacture another one million doses by next weekend.
“According to our estimates, we will be able to produce between 15 and 20 million doses [of Razi Cov Pars vaccine] by the end of the current year,” he pointed out.
Razi Cov Pars is a recombinant protein subunit vaccine containing the COVID-19 spike protein. It reportedly tutors the immune system against the virus by producing antibodies.
The vaccine includes three doses. The first two doses are said to be injectable, whilst the third dose is intranasal.
The second dose of the vaccine will be injected into volunteers 21 after the first inoculation, and the third dose will be inhaled 51 days later.
Last Wednesday, Einollahi wrote on his Twitter account that the campaign against the coronavirus and vaccination is a priority of the new Iranian administration.
He added that imports of coronavirus vaccines will be accelerated within the next few weeks, calling on the people to help the health ministry speed up the process of vaccination.
On June 27, Iran’s homegrown recombinant Noora vaccine, produced by Baqiyatallah University of Medical Sciences, was put on display during a ceremony in Tehran in the presence of Chief Commander of the Islamic Revolution Guards Corps (IRGC) Major General Hossein Salami, Iran’s former health minister Saeed Namaki as well as other Iranian health officials.
Iran has also successfully completed the first phase of the human trial for FAKHRAVAC COVID-19 vaccine developed by the Organization of Defensive Innovation and Research.
It is the third Iranian COVID-19 vaccine reaching clinical trials, currently in the second phase.
FAKHRAVAC is an inactivated virus-based vaccine, and apparently requires two doses given by intramuscular injection 14 days apart.
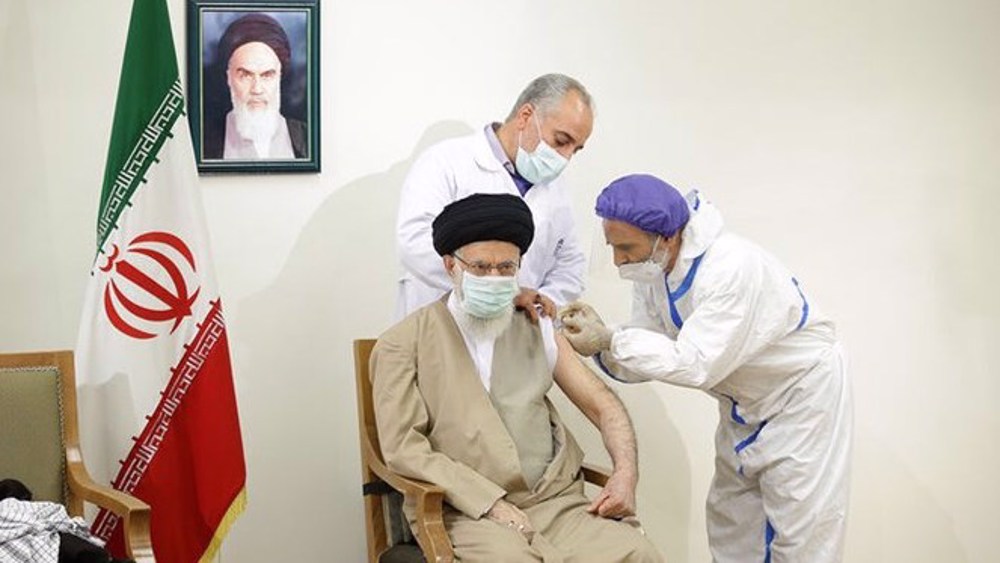
On June 25, Leader of the Islamic Revolution Ayatollah Seyyed Ali Khamenei received his first dose of the CovIran Barekat vaccine, which was approved for emergency mass application by the country’s Health Ministry earlier that month.
After receiving the vaccine, the Leader thanked all those involved in the development of the homegrown vaccine, produced as part of a project led by the Headquarters for the Execution of Imam Khomeini's Order, a charitable organization headed by Mohammad Mokhber.
Ayatollah Khamenei thanked Mokhber and his colleagues as well as Namaki and all of the country’s health workers who have been fighting against the deadly COVID-19 virus for more than a year.
Pezeshkian: Enemies will take dream of Iran’s surrender to grave
US confirms Iran destroyed $300m radar system in Jordan
Imam Khamenei: A legacy forged in unshakable faith, bravery and steadfastness
Iran Navy announces wave of fresh drone strikes on US bases, strategic Israeli sites
Pezeshkian reaffirms Iran’s commitment to defend sovereignty, pursue lasting peace
Many US troops exploring ways to evade fighting in war on Iran: Anti-war activist
Indian journalist reveals severe Israeli censorship after escape from occupied territories
Iran blasts UN chief for downplaying US-Israeli atrocities












 This makes it easy to access the Press TV website
This makes it easy to access the Press TV website